All vitiligo patients in the UK should know about Opzelura avaiability on the market, and the alternatives in the meantime.


All vitiligo patients in the UK should know about Opzelura avaiability on the market, and the alternatives in the meantime.


Understand the similarities and differences between Briumvi, Ocrevus and Kesimpta in one simple overview.


Can you use Jakavi for your vitiligo, while waiting for Opzelura to become available on the European market? All you need to know.

Is it possible to get a Leqembi home infusion? Will you be able to inject Leqembi at home by yourself soon? Learn all we know.


The difference between Leqembi and Aduhelm, simply explained. How do these Alzheimer's medicines work, where are they approved, and how can you access them?

How nitisinone tablets give hope to HT-1 and AKU patients. Now also available for free in India, Pakistan, Bangladesh and Sudan.


Find hope and support with Alzheimer's treatment options. Learn about innovative therapies and resources to manage symptoms and improve quality of life.


Everything you need to know about Briumvi, the latest approved CD20 antibody for the treatment of multiple sclerosis.

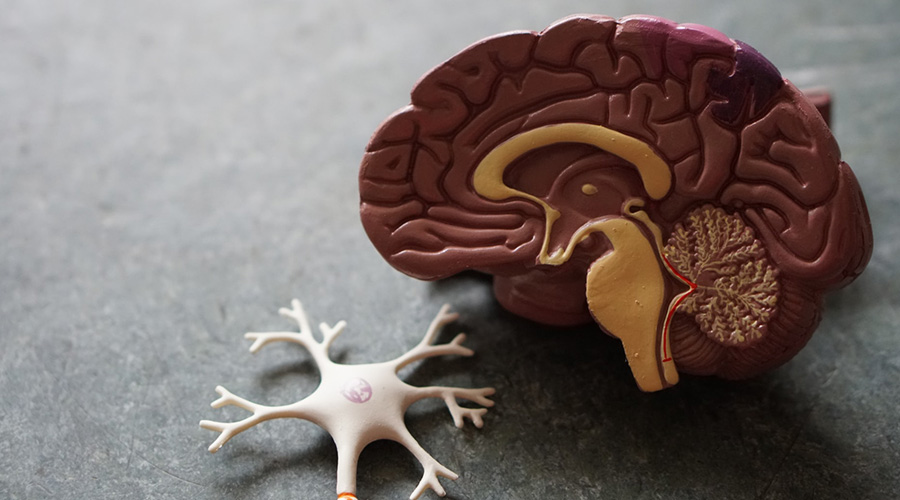
Anti-amyloid therapy for Alzheimer's, explained simply. What is it, how does it work, which one is approved, and how can you access it?

All the safe and legal options to access Leqembi while it's waiting for its EU approval.

All you need to know about Leqembi, the latest approved Alzheimer's medicine.


A simple overview of standard and emerging NSCLC treatments.
Understanding KRAS inhibitors for lung cancer: how they work, which ones are approved, and what's coming?


Sotorasib vs Adagrasib: A side-by-side comparison of the latest Alzheimer's medicines.


Anti-amyloid therapy for Alzheimer's, explained simply. What is it, how does it work, which one is approved, and how can you access it?

Amsterdam's online medicine platform Everyone.org offers affordable cancer medicines for patients in poorest countries thanks to partnership with ATOM coalition in Geneva.
Medicines should be available to everyone, no matter who you are or where you live. At TheSocialMedwork, for the past 7 years, we have been helping patients and their physicians access medicines not yet available where they live.

The Medicine Access Masterclass is a unique overview of early medicine access options of innovative medicine which cannot be found anywhere else.

Paxlovid is an antiviral administered orally to patients who are ill with COVID-19 or have been exposed to the coronavirus SARS-CoV-2 and are at risk of developing severe illness. The manufacturer, pharmaceutical and biotechnology giant Pfizer, developed it to patients avoid severe illness which can lead to hospitalization and death. It is meant to be administered at the first sign of infection.

The SARS-CoV-2 virus is spread through human-to-human transmission. Coughing and sneezing produces small droplets, which can be breathed in by someone else who then becomes infected. The droplets can also be passed on via hand contact, for example if someone touches their nose or face and then shakes hands.
Molnupiravir is an orally administered antiviral medication that inhibits the replication of certain RNA viruses, and is used to treat COVID-19.

Mari Carmen is the older sister of patient Juan Manuel, she takes care of her brother along with his wife. He was diagnosed with ALS in February 2020 and since then he has followed a clinical trial that includes Ketas (Ibudilast) which is not yet approved in Spain, where Juan Manuel lives.

Non-Hodgkin lymphoma (also called non-Hodgkin's lymphoma, NHL, or sometimes just lymphoma) is a type of cancer that starts in white blood cells known as lymphocytes. These cells are part of the body's immune system and they help the body fight infections.

Mislav was diagnosed with Grade II astrocytoma, a type of brain tumour, in 2018 and has struggled with the Croatian healthcare system to obtain a treatment, Fycompa, which was not approved in Croatia. TheSocialMedwork was able to help him access the medicine and he is now one of the patients that we are happy to not have anymore: the hospital where he is being treated has approved the import and treatment of Fycompa to him for free.

Bronchial asthma (also known as asthma) is a pulmonary disease, in which the airways narrow, swell and produce more mucus than necessary.

Iron deficiency is a state where the body lacks iron. It is the most common nutritional disorder in the world.

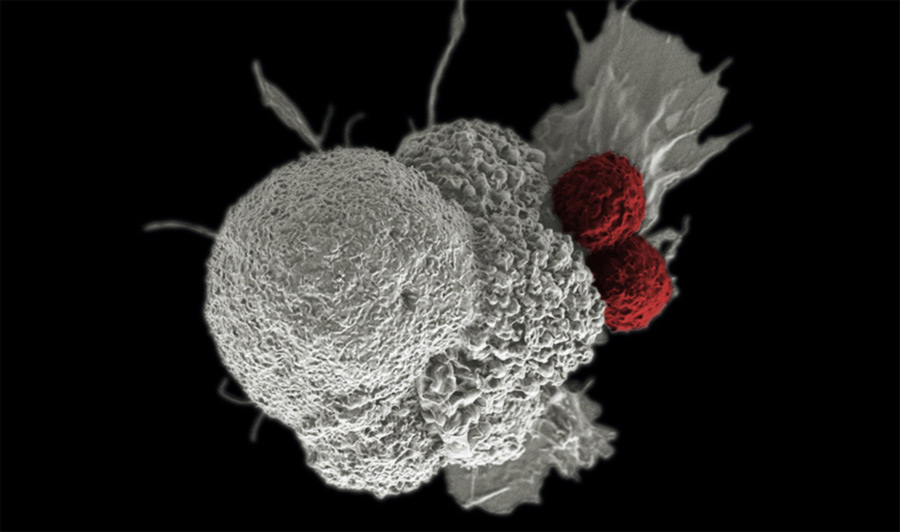
Multiple myeloma is a type of bone marrow cancer. Bone marrow is the soft tissue inside bones that produces the body's blood cells, including a type of white blood cell called plasma cell. Plasma cells play an important role in the immune system: they produce antibodies that help the body fight off germs.
Neuroblastoma is a type of cancer that is formed by nerve cells called neuroblasts. Usually, these very early forms of nerve cells develop into adult nerve cells, but in neuroblastoma these cells develop into cancer cells instead. This type of cancer occurs most commonly in infants and young children. Neuroblastoma normally forms in the fetus, before a child is born.
Bladder cancer - or urothelial cancer - most often begins in the cells that line the inside of the bladder, called urothelial cells. Urothelial cells are also found in other parts of the urinary system, such as the kidneys and the ureters. Urothelial cancer is found much more often in the bladder than in the other parts. But because people sometimes have tumors in these places, too, all of the urinary tract needs to be checked for tumors.

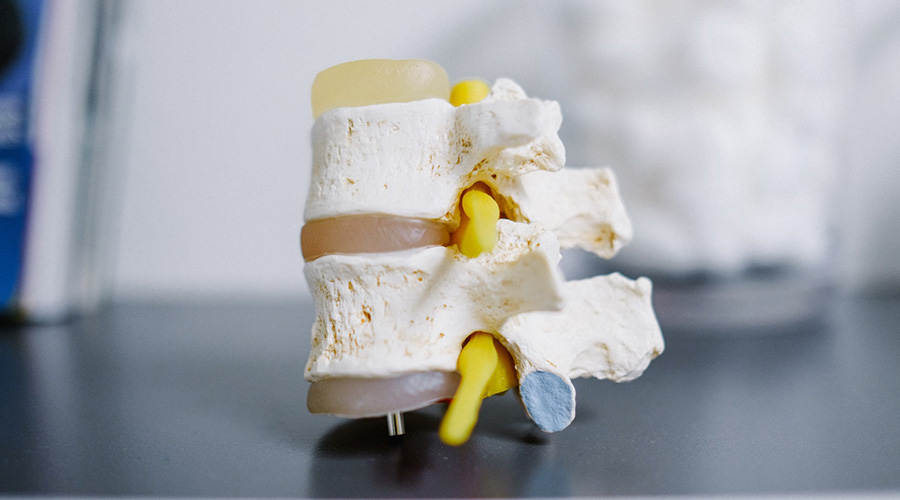
Spinal Muscular Atrophy (SMA) is an inherited disorder that affects nerves and muscles, which causes muscles to become increasingly weak. The cause is a shortage of the protein SMN, or ‘survival of motor neuron’. This protein is crucial for the function of nerves that control muscle activity. Without this protein, the motor neuron cells shrink and eventually die. This leads to muscle weakness seen in SMA..